1. Introduction
2. Materials and Methods
2.1 Characterization of soil and amendments
2.2 Experimental set-up
2.3 Contaminant extractability leachability, and bioaccessability
2.4 Soil enzyme activities
2.5 Phytoavailability
2.6 Quality control
2.7 Statistical analyses
3. Results
3.1 Soil pH and EC
3.2 Contaminant extractability, leachability and bioaccessability
3.3 Soil enzyme activity
3.4 Plants
3.5 Correlations
4. Discussion
5. Conclusion
1. Introduction
Soil plays important roles in supporting primary productivity, modulating nutrient flows, and promoting the ecosystem health (Xian et al. 2012). Toxic trace elements (TTEs) in soils derived from human activities are constantly released into the terrestrial environment and therefore they are of increasingly growing concern worldwide (Wahsha et al. 2016). People are becoming more aware of the inferences of TTEs contaminated soils on human and environmental health, resulting in the improvement and development of technologies for clean-up of TTEs contaminated sites.
Various techniques can be used to decrease the risk posed by toxic elements in mined soils. The selection of specific technological methods is dependent upon the extent and nature of the contamination, type of soil, characteristics of the contaminated site, availability of materials, and relevant regulations. Conventional techniques to remediate TTEs contaminated soils are mainly based on physical and chemical methods. Despite high efficiency, majority of these techniques are costly, environmentally destructive. Financial and technical implications and complexities have made soil clean-up a challenging task. Innovative low-cost, low-input technologies are needed for soil remediation and community acceptance. One promising technology is the in situ stabilization of TTEs in soils by the addition of various amendments (Oste et al. 2002, Basta and McGowen 2004). Compared with other remediation techniques, in-situ chemical stabilization is less expensive and may provide a long-term remediation.
A continuous monitoring of a contaminated site is a mandatory need mainly when a polluted site has to be characterized for a possible further design and implementation of a restoration process. It must be verified whether the cleanup of the site has been effectively achieved and the pollution completely removed. The recovery of derelict soils should be assessed not only by soil chemical characteristics determined by conventional analytical tests and extraction procedures, but also by assays that measure the restoration of soil habitat function. In fact, chemical data alone are not sufficient to evaluate the toxic effects of pollutants and characterize contaminated environments, as they do not consider the effects of chemical compounds on organisms or the interactions among contaminants, the soil matrix, and biota (Brown et al. 2005, Leitgib et al. 2007). It is difficult to determine the point at which degraded soil is fully restored. Thus, indicators that reflect the state and function of the soil, such as soil enzyme activities, and link information regarding microbial biomass and soil physical and chemical parameters are indispensable (Leitgib et al. 2007, Antunes et al. 2011).
Soil enzymes play critical roles in organic matter decomposition, redox reactions and nutrient cycling. Their activities indicate the degree of biochemical reactions in soil, and can serve as important biological indicators for evaluating quality of soil contaminated by TTEs (Tang et al. 2019). In other words enzymes may act as sensors of a specific molecule or its by-products, may evaluate their amounts and concentration and assess the effective restoration of a contaminated site. In this case they act as biosensors and may provide simple, highly sensitive assays to detect initial indication of either environmental pollution or obtained restoration (Garcia et al. 2001, Kudanga et al. 2011, Van Dyk and Pletschke 2011). They may give information not only on the impact of pollution on the quality of the involved environment but also on its actual restoration by a remediation treatment (Niemeyer et al. 2012, Gao et al. 2013).
Important role that enzymes play in the environment is to assist the detection and quantification of the amount of pollutants and to give indications on the health and quality status of the polluted/restored target environment. In other words they may act as (bio) sensors and (bio) indicators (Rao et al. 2014). However, to our knowledge, little information is available about the effects of amendments addition on enzyme activities in soils contaminated TTEs. The relationships between enzyme activities and physicochemical properties, (bio) availability of the TTEs have been rarely evaluated simultaneously. We assessed the ability of several industrial byproducts (furnace slag and red mud) to reduce the availability of TTEs in soils and evaluated the response of soil enzymes as biological indicators to the soil amendments.
2. Materials and Methods
2.1 Characterization of soil and amendments
Used soil in this investigation was collected from agricultural land adjacent to the abandoned Dukgog gold mine in Chungnam Province, Korea (36°37'44"N, 126°56'58"E). The physico-chemical properties of the soil (<2 mm) and amendments were shown in Table 1. Textural analysis was performed using the pipetting method of Gee and Bauder (1986). Soil pH and electrical conductivity (EC) were determined using 1:5 soil/H2O (Thomas 1996). Total C and N were determined by the Turin (Nelson and Sommers 1996) and Kjeldahl (Mremner 1996) methods, respectively. Cation exchange capacity (CEC) was determined using the ammonium-saturation and distillation method (Sumner and Miller 1996). Pseudo-total trace element (As, Cd, Cu, Pb, and Zn) concentrations in soils and amendments were measured by inductively coupled plasma optical emission spectrometry (ICP-OES) after digestion of the samples with aqua regia according to ISO 11466 (ISO 1995). Table 1 shows the main physico-chemical properties of both the soil and amendments used in this investigation.
Table 1.
Soil physic-chemical properties and total metal concentrations of the soils and amendments used in this experiment*
2Particle size was analyzed by pipetting method.
3Cation exchange capacity.
4Aqua regia extractable metal concentration.
5“-” means not determined.
2.2 Experimental set-up
Furnace slag and red mud were added to Dukgog mine soil (arsenic contaminated soil) at 2 - 6%, and 1 - 4% w/w rate. Amendments used in this experiment were reduced to a particle size of <2 mm. Non-amended soil was used as the control. The amendments were thoroughly mixed with the soils to obtain homogeneity, and were then equilibrated for 40 d, during which soil moisture was maintained, and the soils were thoroughly mixed at weekly intervals.
2.3 Contaminant extractability leachability, and bioaccessability
The effect of each amendment on contaminant extractability was evaluated using 0.1 M Ca(NO3)2. Extraction with 0.1 M Ca(NO3)2 solution was performed according to the procedure of Conder et al. (2001) to measure Ca(NO3)2-extractable metals: 1 g soil was combined with 0.1 M Ca(NO3)2, mixed in a shaker, centrifuged for 20 min at 5100 × g, and then filtered through Whatman GF/F 0.7 µm borosilicate glass filters. Contaminants were measured in soils by TCLP extraction according to the USEPA method 1311 (USEPA 1986). The TCLP extracting agent was acetic acid (pH 2.88). The solid/liquid ratio TCLP was kept at 1:20. After 18 h of mixing in a TCLP tumbler, the leachate was filtered using Whatman GF/F 0.7 µm borosilicate glass filters. The availability to the human gastrointestinal (GI) system of TTEs in ingested soil was estimated using the modified Physiologically Based Extraction Test (PBET) procedure described by Geebelen et al. (2003): 0.35 g soil was shaken (30 rpm) with synthetic gastric solution (0.4 M glycine; pH 2.2) for 1 h at 37°C, and then filtered through Whatman GF/F 0.7 µm borosilicate glass filters. The filtrate was acidified by adding a small amount of nitric acid to pH< 2, followed by ICP analysis.
2.4 Soil enzyme activities
Immediately following aging, soils were subsampled to determine enzyme activity and soil microbial biomass, which were used as microbial endpoints. Soil subsamples were kept moisture at under 4°C. Before analyses, samples were sifted through a 2 mm sieve, and their water content was determined to express microbial activity on a dry-matter basis. Dehydrogenase activity was determined by the reduction of 2,3,5-triphenyltetrazolium chloride (TTC) to triphenyl formazan (TPF) (Casida et al. 1964). Phosphatase activity was determined using p-nitrophenyl phosphate disodium as a substrate (Tabatabai and Bremner 1969) and urease activity was measured using the method of Kandeler (1995). Although fluorogenic substrate assay has been widely used due to high sensitivity and potential for simultaneous assay of multiple enzyme in the same samples, it also raised concerns about data accuracy and reproducibility (Zhang et al. 2010, Deng et al. 2017). For this reason, chromogenic enzyme assays were conducted.
2.5 Phytoavailability
To examine the effect of the addition of amendments on the phytoavailability of contaminants, lettuce (Lactuca sativa. L) was grown in plastic pots (1 kg capacity, 15 cm diameter, 25 cm height) filled with contaminated soils, prepared as described above. Lettuce seeds were initially sown in peat-based horticultural compost. 2 wk after germination, the seedlings were transplanted into contaminated soils (5 seedlings/pot, 3 pots per treatment). The trials were conducted under controlled greenhouse conditions (temperature 15 - 25°C, relative humidity 60 - 70%) with daily watering. After 45 d, the lettuce was harvested, rinsed with DW, and dried at 80°C for 48 h. Lettuce shoot samples were digested in hot nitric acid, and the resultant solutions were filtered.
2.6 Quality control
All chemicals were of analytical grade or better. Centrifuges were acid washed (5% HNO3) before each use, and disposable labware was used for all other analyses. Heavy metals analyses were conducted using inductively coupled plasma optical emission spectrometry (ICP-OES); blanks were run for background correction and other error sources. Multi element standards were run every 20 samples, with recovery within 100 ± 10%.
2.7 Statistical analyses
All values obtained from soil chemical and biological analyses were reported as the means of three replicates. The data were checked for homogeneity of variance and normality (Kolmogorov-Smirnov test) and, when possible, subjected to one-way ANOVA. For significant differences (p < 0.05), a post-hoc Tukey honestly significant difference (HSD) test was used to further elucidate differences among means (p < 0.05). Pearson correlation coefficients were calculated between soil metal contents and microbial activity and plant metal contents. Statistical analyses were conducted using SPSS 11.5 for Windows.
3. Results
3.1 Soil pH and EC
After amendments were applied, both pH and EC increased significantly (p < 0.05; Table 2). Soil pH increased from 6.54 to approximately 7.81 and 8.64 in 6% furnace slang - and 4% red mud-amended soil, respectively. Soil EC increased from 238 to 339 and 369 µS cm-1 in the furnace slag and red mud treatments, respectively.
Table 2.
pH and electrical conductivity (EC) of the soils with different amendment additions*
1FS, furnace slag; RM, red mud.
3.2 Contaminant extractability, leachability and bioaccessability
The application of amendments had no significant effect (p > 0.05) on total As, Cd, Pb, and Zn concentrations in soils (data not shown). We determined contaminant’s extractability, leachability, and bioaccessibility. The application of amendments also significantly decreased Ca(NO3)2-extractable TTEs concentrations, with red mud being the most effective (Table 3). Compared to the control soil, a 4% red mud addition reduced Ca(NO3)2-extractable As, Cd, Cu, Pb, and Zn by 28%, 92%, 78%, 100%, and 100%, respectively. Contaminants leachability was also significantly reduced by amendments addition, with red mud being the most effective (Table 4). Compared to the control soil, a 4% red mud addition reduced TCLP-extractable As, Cd, Cu, Pb, and Zn by 44%, 42%, 82%, 97%, and 60%, respectively. Bioaccessible soil TTEs was determined using the PBET method (Table 5). PBET Pb decreased significantly only in the 4% red mud treatment, which resulted in 29%, 10%, 50%, and 4% of bioaccessible As, Cd, Pb, and Zn, respectively.
Table 3.
Ca(NO3)2 extractable toxic trace elements (mg kg-1) of the soils with different amendments addition*
1FS, furnace slag; RM, red mud.
Table 4.
Leachability (TCLP) toxic trace elements (µg kg-1) of the soils with different amendments addition*
1FS, furnace slag; RM, red mud.
Table 5.
Physiologically based extraction test (PBET) toxic trace elements (mg kg-1) of the soils with different amendments addition*
1FS, furnace slag; RM, red mud.
3.3 Soil enzyme activity
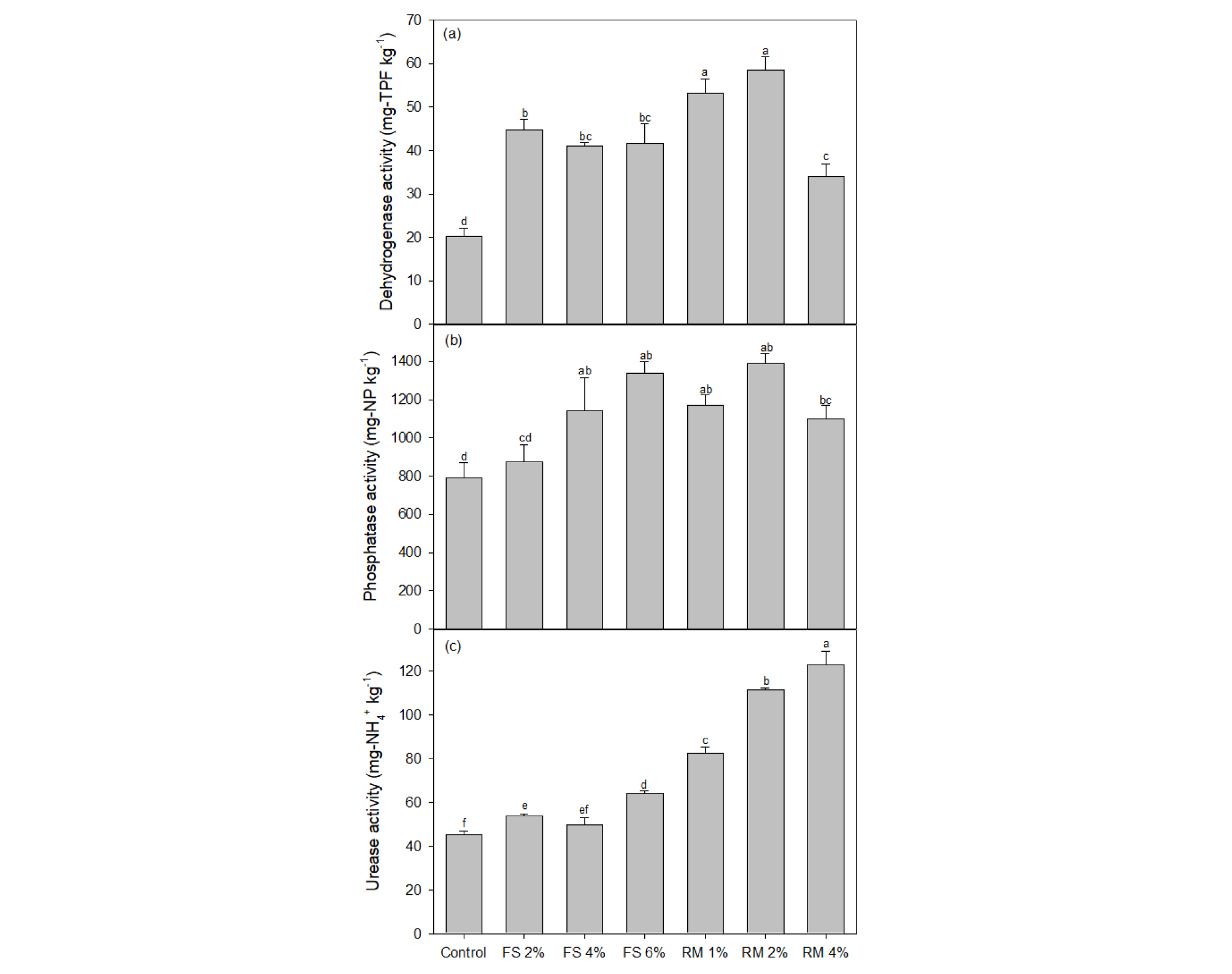
Compared to non-amended soil, dehydrogenase, phosphatase and urease activity were significantly higher, in furnace slag- and red mud-amended soils (p < 0.05; Fig. 1). But activity of dehydrogenase and phosphatase were decreased in highest ratio red mud (4%) amended soil. Urease activity was continuously increased with increasing the amount of amendment. Usually, higher enzyme activities were observed in red mud - amended soils than furnace slag - amended soils.
3.4 Plants
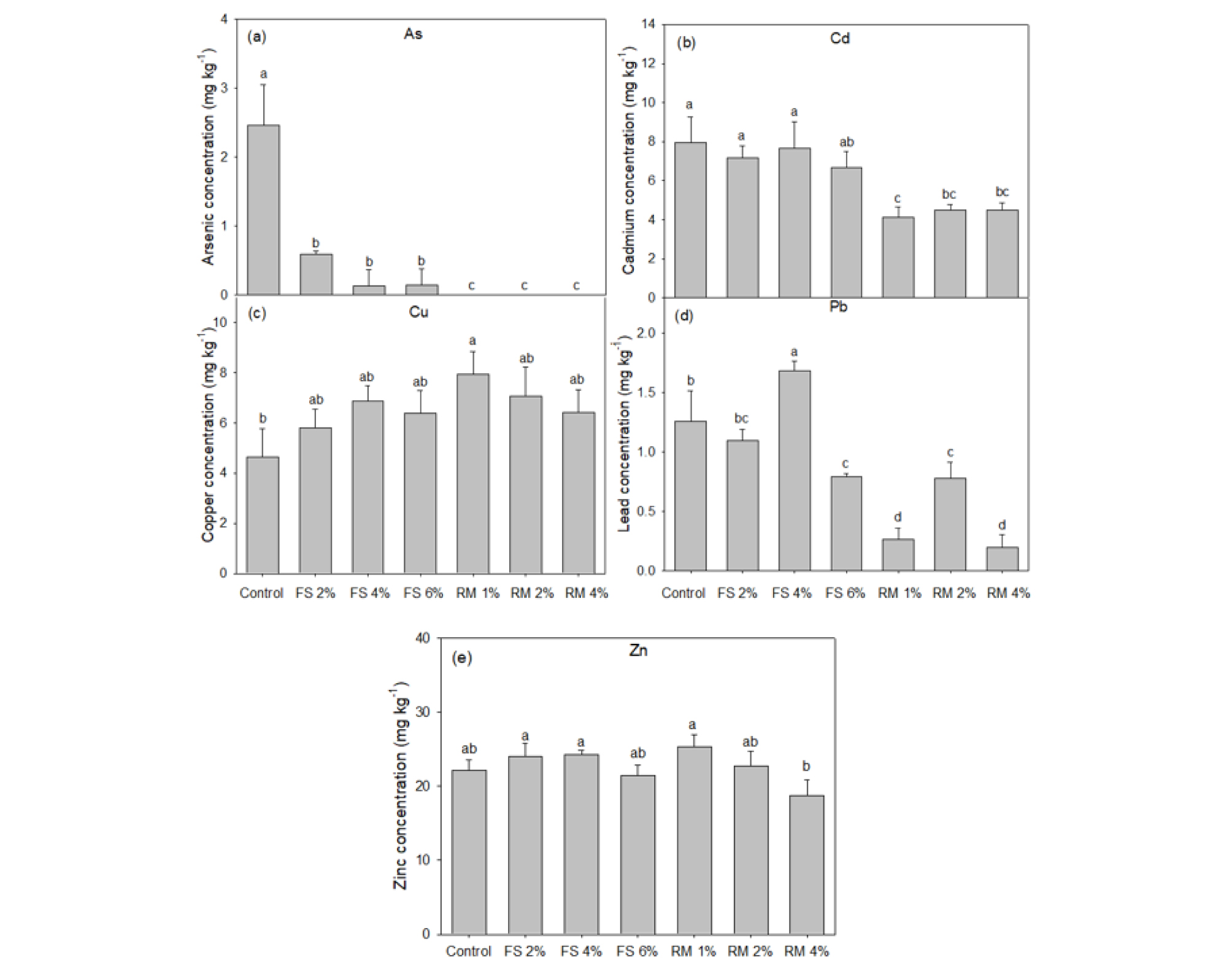
Compared to non-amended soil, the concentrations of TTEs in lettuce grown in red mud-amended soils showed significant (p < 0.05) decreased (Fig. 2). Most notable was that grown in soil amended with 4% red mud which had 0%, 57% and 16% of the As, Cd and Pb, respectively, detected in lettuce shoots grown in control soil. The effects of amendment addition on the reduction of Cu and Zn translocation were not significant.
3.5 Correlations
Table 6 shows the Pearson’s correlation coefficients between extractable TTEs, soil enzyme activities, and lettuce bioconcentrations. Significant negative correlations were observed between TTEs contents and soil enzyme activities. The correlation coefficients were significantly higher between soil enzyme activities and Ca(NO3)2-extractable TTEs contents than TCLP and PBET- extractable. Significantly higher correlations were observed between Ca(NO3)2- extractable Cd, leachable Cd and Pb, bioaccessible Pb and lettuce concentrations.
Table 6.
Correlation coefficients between TTE concentrations and soil enzyme activities in soils (n = 21)
1DHA, dehydrogenase; PME, phosphatase; URE, urease; EC, electrical conductivity.
4. Discussion
In this study, we compared the ability of two kinds of amendments to reduce TTEs availability. Amendments, furnace slag and red mud are all industrial waste or by-products which have been shown to have great potential in TTEs stabilization in soil. The application of amendments that can immobilize TTEs in situ may provide a cost-effective and sustainable solution for the remediation of contaminated soil (Oste et al. 2002, Lee et al. 2011). The total concentrations of TTEs in soil did not change significantly when amendments were added (data not shown). Although the amendments had no effect on total metals concentrations, they significantly decreased the available fractions of TTEs (Tables 3 - 5). It can be said that the stabilization of TTEs in this experiment might be resulted from two processes. First, the increased soil pH associated with the application of alkaline amendments reduced solubility of TTEs in soil and increased metal sorption to soil particles because of the net negative charge increase of variably charged colloids, such as clays, organic matter, and Fe and Al oxides (Kumpiene et al. 2008). Also, increased soil pH and carbonate buffering can lead to the formation of metal-carbonate precipitate complexes that decrease metal availability. Second, the availability of soil TTEs are mainly controlled by adsorption/desorption processes and co-precipitation (Kumpiene et al. 2008). The great reduction in availabile TTEs was likely due to their sorption via inner sphere complexation on the reactive surface of newly formed Fe oxides. Fe oxides have the greatest potential for As and heavy metal adsorption in soil (Kumpiene et al. 2008) the large amount of Fe and Al oxides in furnace slang and red mud introduce new sorptive surfaces that may immobilize heavy metals in soils through specific sorption or chemisorption (Lee et al. 2011, Kim et al. 2020).
The significant decrease of Ca(NO3)2-extractable TTEs concentration, which represent TTEs that are sorbed onto the soil solid phase (McLaughlin et al. 2000). The decreased concentrations of extractable TTEs in the amended soils can be attributed in part to a significant increase in soil pH (Table 2) caused by the alkaline nature of the amendments. The Ca(NO3)2 extracts used as surrogate measures of TTEs availability were non-aggressive extracts developed to measure readily labile metals in soils (Lee et al. 2011). Unlike Ca(NO3)2-extractable TTEs contents, the effects of amendments on bioaccessible TTEs, determined using the PBET, were minimal (Table 4), which may be attributed to the difference in the extractant pH. The acidic (pH 2.2) extraction solution of the PBET is more aggressive at dissolving TTEs than is a neutral solution (pH 7.0; Ca(NO3)2). Thus, TTEs is easily desorbed and solubilized by the low pH of the GI tract (Geebelen et al. 2003).
Soil enzyme activities were negatively correlated with content of available TTEs fraction. Our results were consistent with several other studies demonstrating that soil enzyme activities are inversely related to TTEs concentrations (Madojón et al. 2001, Karaca et al. 2002, Moreno et al. 2002). We found strong negative correlations between Ca(NO3)2-extractable TTEs content and soil enzyme activities. The inhibition of enzyme activities by toxic elements in soil is principally attributed to an indirect effect (suppression of the microbial population and cellular activities) and/or a direct effect (inactivation of extracellular enzymes due to binding with the metals) (Kizilkaya and Bayrakli 2005).
Dehydrogenase, phosphatase and urease activity has a positive correlation coefficient of 0.4415, 0.5187 and 0.7165, respectively with soil pH, indicating the importance of the pH on the enzyme activities. A similar relationship was also reported by Mora et al. (2005). At the same time, the increase in pH implies a reduction of the available TTE fractions, which in turn reduces heavy metal toxicity to microorganisms. The positive effect of soil amendments on soil enzyme activities may be attributed to a decrease in the bioavailability of TTEs, as estimated by the soil extractions. The observed positive correlation between soil enzyme activity and the pH indicates the beneficial effect of the amendments on soil pH. Similarly, the increase in EC induced by additives significantly activated most of soil enzyme activities may indicate a positive effect of EC on enzyme activities. Ellis et al. (2001) found that pH had the greatest bearing on dehydrogenase activity in a Zn-polluted soil. Dehydrogenase activity negatively correlated with Cd (p < 0.05), and Zn (p < 0.01) soluble concentrations (Table 6 and Fig. 1). Dehydrogenase is widely used in evaluating the metabolic activity of soil microorganisms. Dehydrogenase has been used as an indicator of microbiological activity in soil management studies (Bergstrom et al. 1998). Dehydrogenase activity depends on the metabolic state of soil microorganisms although its use as an indicator of soil microbial activity has been criticized because the enzyme is affected by numerous factors (soil type, pH, etc.) (Nannipieri et al. 1990). Dehydrogenase could be determined from the intracellular enzymes in a reduced number of microbes in soil or could be the result of differences in the composition of microbiota in stressed soil. Some researchers (Oliveira and Pampulha 2006, Kim et al. 2018) suggested that dehydrogenase assay is a useful indicator for evaluating the effect of toxic metals on soil microbial activity.
Though, examined enzyme in this experiment showed similar response to soil management, the response of soil enzymes to soil parameters has not yet reached a consensus conclusion due to the complex environmental conditions (Tang et al. 2019). There are many differences in the existing literature on the relationship between soil physicochemical parameters and soil enzymes. For example, Huang et al. (2017) proved that pH was negatively correlated with invertase and alkaline phosphatase activities, and positively related to urease activity, but Yang et al. (2016) found that these enzyme activities were independent of pH, and Bera et al. (2016) showed that a positive correlation between pH and alkaline phosphatase activity.
The decreased uptake of TTEs by lettuce grown in amended soils is clearly related to the decrease of the available fraction of TTEs. The most obvious effect was the significant reduction of TTE transfer in lettuce grown in soil amended with red mud (Fig. 2). Lee et al. (2011) also reported that heavy metals content in lettuce shoots was greatly reduced by red mud treatment. The decreased uptake of As and metals observed in this study was clearly related to the decrease in available fractions of As and metals (Fig. 2). The comparable literature report is that of Ascher et al. (2009). They reported that As contents in lettuce shoot were largely reduced by iron containing amendments and foliar As, Cd, Pb, and Zn concentrations in lettuce shoots correlated closely with concentrations in soil extracts.
5. Conclusion
Our results point to the potential use of soil enzyme activities as indicators of soil quality changes associated with TTEs and remedial measures. Thus, monitoring the change of soil enzyme activities are useful indicators of the re-establishment of biotic connections and restoration functions in degraded systems. But the impact of soil properties on enzyme activities of TTEs-contaminated soils is still the direction of future research, especially in the presence of additives.